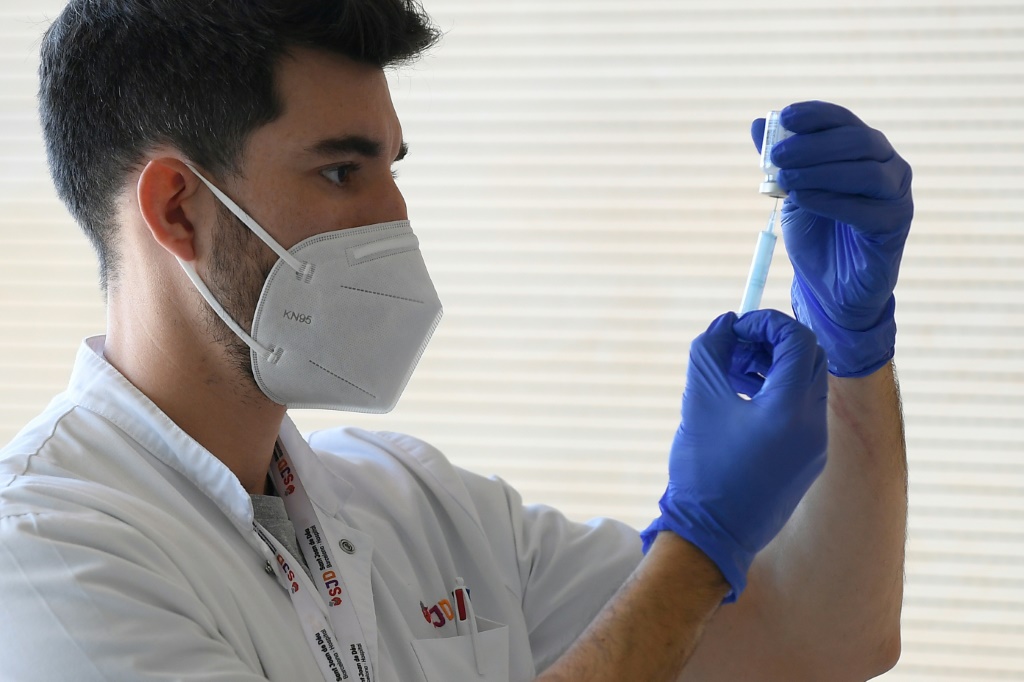
The Centers for Disease Control and Prevention (CDC) has made some changes to its vaccine guidance after factoring in the risk of myocarditis among those jabbed with Pfizer and Moderna vaccines.
Myocarditis Risk
Previously, the CDC indicated on its website that the second dose of the mRNA vaccines from Pfizer and Moderna should be administered three to four weeks after the first dose. To be exact, Pfizer recipients were advised to get the second dose after three weeks, while Moderna recipients were told to get the follow-up dose after a month.
But earlier this week, the public health agency tweaked its guidance, stating that certain people should get their second dose after eight weeks. The reason, according to the CDC, was the risk of developing myocarditis — a type of heart inflammation — in some people.
The amendment in the guidance came after cases of myocarditis were reported after some people received their second mRNA vaccine dose. Medical professionals even said that the condition was most common among males ages 12 to 29.
Citing scientific data from multiple sources, the CDC said the Moderna vaccine displayed a higher risk for heart inflammation compared to the Pfizer vaccine. Most of the people who developed the condition even had to be hospitalized for short periods to help with the acute symptoms.
Guidance Update
The new guidance posted on the CDC’s website states, “An 8-week interval may be optimal for some people ages 12 and older, especially for males ages 12 to 39 years.” The agency explained that the risk could be reduced by extending the interval between the doses.
“Eight weeks can create the opportunity to develop stronger and more broad immunity, which could be important in future waves of the pandemic,” Matthew Tunis, executive secretary for Canada’s National Advisory Committee on Immunization, told CNN.
It is important to note that this change does not apply to all. The CDC maintained that the vaccines remain safe and effective at their original intervals. The original vaccination guidance would still be followed for those who are moderately or severely immunocompromised and for adults 65 and above who need “rapid protection” from the novel coronavirus due to community transmission and severe disease risks.
Dr. Sara Oliver, an epidemic intelligence service officer with the CDC’s Division of Viral Diseases, said the “benefits for both mRNA vaccines far outweigh the risk of myocarditis compared to no vaccine.”