A new blood test could soon provide an accurate and rapid means to screen and diagnose prostate cancer.
Researchers at the University of East Anglia presented their new method of detecting prostate cancer in a new study published in the journal Cancers.
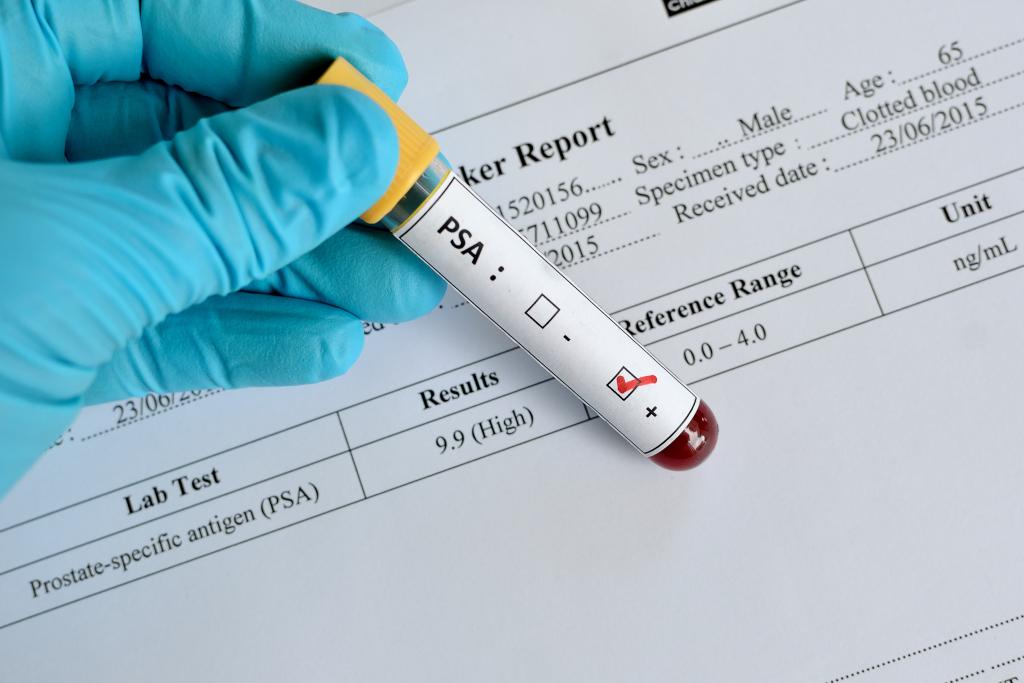
The team developed an epigenetic test that detects cancer-specific chromosome conformations in the blood. They then combined the test with the prostate-specific antigen (PSA) blood test to see if the combo, dubbed Prostate Screening EpiSwitch or PSE test, could yield more accurate results.
“Our results demonstrate that the new combined test (termed PSE test) allows significant increase in prostate cancer detection compared to PSA or epigenetic test alone. This new PSE test is accurate, rapid, minimally invasive, and inexpensive. If successful in larger trials, it may significantly improve prostate cancer diagnosis,” the team wrote.
Individually, the tests had different accuracy levels. But together, the epigenetic test and PSA blood test resulted in a 94% accurate diagnosis, beating the accuracy levels of current methods in prostate cancer diagnosis.
In a statement obtained by News Medical, UEA’s Norwich Medical School professor Dmitry Pshezhetskiy noted that there is no single test for prostate cancer available at present. Doctors use PSA blood tests, physical examinations, MRI scans and biopsies to diagnose patients.
He added that PSA blood tests are not routinely used to screen patients because the results they yield are not entirely reliable. This issue drove Oxford BioDynamics, the UEA, Imperial College London and Imperial College NHS Trust to develop the PSE test.
The pilot study evaluated the new test for accuracy in 147 patients. All of the men had prostate cancer, and the test was 94% accurate in diagnosing them. For the next phase of research, the team wants to evaluate the PSE test on a group of men with unknown cancer status, according to the Independent.
“There is a clear need in everyday clinical practice for a highly accurate blood test that can screen men for prostate cancer and accurately identify those at risk, while sparing those who up to now would be subject to unnecessary, expensive and invasive procedures,” Oxford BioDynamics CEO Dr. Jon Burrows said, as per News Medical.
“This is another example of how our product portfolio can contribute to reducing the total cost of care for global health,” he added.
Prostate cancer is the most common cancer in American men, aside from skin cancer. The American Cancer Society estimates that in 2023, the U.S. could record about 288,300 new cases and around 34,700 deaths.