Weight loss drugs have gained a lot of popularity in recent times, and many people have jumped on the bandwagon without giving the trend a second thought. However, there are potential negative effects associated with the medication that people should know about.
It all started when researchers found that a class of drugs used to treat type 2 diabetes also contributed to weight loss. As a result, drugs, such as Victoza and Ozempic, became popular treatment options for obesity.
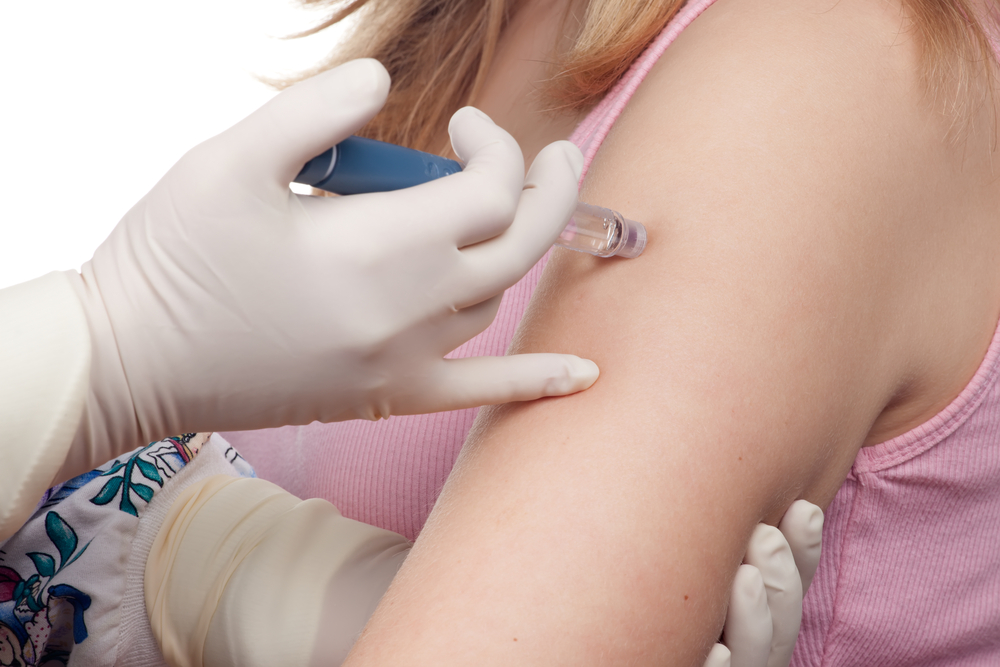
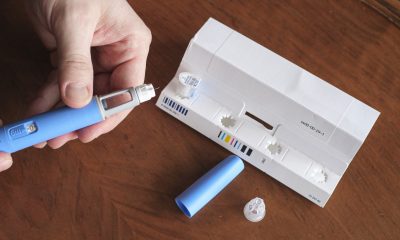
Called GLP-1 Ras, these drugs are administered as daily or weekly injections. The medication helps the body produce insulin and lower blood sugar levels. The drugs were approved for use in type 2 diabetes patients in 2005 by the U.S. Food and Drug Administration (FDA), as per ABC News.
After its weight loss benefits came to light, the FDA approved GLP-1 RA for chronic weight management in 2014. Subsequently, additional drugs in the class were approved for weight loss.
According to the Journal of the American Medical Association, more than one in 10 of 35 million Americans with type 2 diabetes were estimated to be on this medication in 2019.
“I prescribe these medications 10 times per day,” Dr. Amanda Velazquez, Director of Obesity Medicine at Cedars-Sinai Medical Center, told ABC News. “Obesity is a chronic relapsing disease.”
Velazquez added the weight loss effects of the medications stop after drug disuse.
A recent study from the University of Montpellier published in Diabetes Care found a link between the long-term use of these drugs and the chances of developing thyroid cancer. People, who were on the drugs for 1-3 years, were 58% more likely to get thyroid cancer, the study found. Moreover, the risk for medullary thyroid cancer, a rare form of the disease, was even higher.
“The newer findings provide interesting additional data to this clinical discussion, though are not independently enough to set a new standard for screening,” Dr. Erik K. Alexander, Chief of the Thyroid Section in the Division of Endocrinology, Diabetes, and Hypertension at Brigham and Women’s Hospital, told the news outlet. “[These drugs] should only be used when the benefit of treatment outweighs known or suspected risk, and this assessment should be continually reconsidered by each patient with their physician on a regular basis.”
Novo Nordisk, the pharmaceutical company that makes Ozempic, Victoza, and another drug specifically meant for weight loss called Wegovy, said in a statement that extensive data from trials and real-world evidence “have not shown a causal relationship between the use of GLP-1 receptor agonists and risk of thyroid tumors.”
Currently, patients on these medications are asked every 3-4 months for liver, diabetes, kidney, cholesterol, and electrolyte testing. Thyroid testing is not part of that list.
“The data on thyroid cancer certainly gives me pause,” Dr. Heather Sateia, Assistant Professor of Medicine at Johns Hopkins Hospital, said. “There is not currently a recommendation for thyroid ultrasound or serum calcitonin monitoring, but we are keeping an eye out for changes in those recommendations. I suspect we’ll see a shift in this soon.”