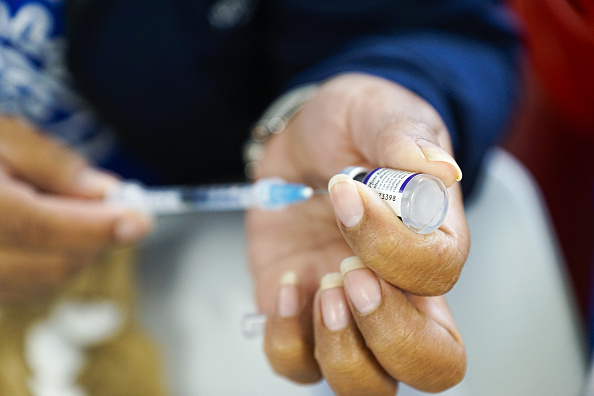
The U.S. Centers for Disease Control and Prevention (CDC) is still looking into the reported stroke risk of older adults who received Pfizer’s bivalent booster shots.
Health officials from the agency gave an update Thursday on the issue, which was brought to light earlier this month, saying the CDC’s investigation into Pfizer’s updated booster is still ongoing, Reuters reported.
Dr. Nicola Klein of Kaiser Permanente, the healthcare company maintaining CDC’s Vaccine Safety Datalink (VSD) real-time surveillance system, said the stroke rate observed in the database had slowed in recent weeks.
According to Klein, the latest reported signal appeared weaker than what the agency flagged two weeks ago. However, it was still statistically significant, meaning the reported cases did not happen by chance.
Klein said most of the confirmed stroke cases received a flu vaccine at the same time — something that should be taken into consideration.
U.S. Food and Drug Administration (FDA) officials, who said they did not detect a link between the shots and strokes in other monitoring databases, want to look into what Klein pointed out.
The FDA plans to study if receiving two booster shots at the same time could increase the risk of stroke, said scientist Richard Forshee.
When CDC brought up the potential safety issue with Pfizer’s bivalent vaccine in mid-January, the agency maintained that it’s very unlikely for the detected safety signal to translate to true clinical risk since no other safety systems reported a similar finding.
“These strokes are not a confirmed adverse event at the moment. It’s like a radar system. You’re getting a blip on the radar, and you have to do further investigation to discover whether that airplane is friend or foe,” Dr. William Schaffner said at the time.
Schaffner is an infectious disease expert at Vanderbilt University and a member of the CDC Advisory Committee on Immunization Practices’ COVID-19 Vaccine Work Group.
Despite the issue, both the CDC and FDA continue to recommend the bivalent boosters to older adults since they target the different coronavirus variants, including the newer omicron strains.
In an email to Reuters, University of Pittsburgh professor Dr. Walid Gellad approved of the two agencies’ move to investigate the safety issue.
“Sometimes signals are not clear. It makes sense to look into it more, and it doesn’t make sense to change practice given the known benefits (of getting the booster) in this age group,” Gellad said.